Genentech to Present First Clinical Data on Novel Anti-TIGIT Cancer Immunotherapy Tiragolumab at ASCO
South San Francisco, CA -- May 13, 2020 -- Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced positive results from the Phase II CITYSCAPE trial, the first randomized study evaluating the efficacy and safety of tiragolumab plus Tecentriq ® (atezolizumab) compared with Tecentriq alone as an initial (first-line) treatment for people with PD-L1-positive metastatic non-small cell lung cancer (NSCLC). Tiragolumab is a novel cancer immunotherapy designed to bind to TIGIT, an immune checkpoint protein expressed on immune cells. Both TIGIT and PD-L1 play an important role in immune suppression, and blocking both pathways could enhance anti-tumor activity. The full results will be presented in an oral abstract session (Abstract #9503) at the ASCO20 Virtual Scientific Program organized by the American Society of Clinical Oncology (ASCO), which will be held May 29-31, 2020.
“We are pleased to share these first randomized anti-TIGIT results, showing that tiragolumab, our novel cancer immunotherapy, has encouraging efficacy and safety in combination with Tecentriq,” said Levi Garraway, M.D., Ph.D., chief medical officer and head of Global Product Development. “TIGIT, an immune checkpoint protein expressed on immune cells, was identified by our own scientists. By blocking both TIGIT and PD-L1 pathways simultaneously, we hope to deepen patient responses to immunotherapy and widen the circle of people who may benefit.”
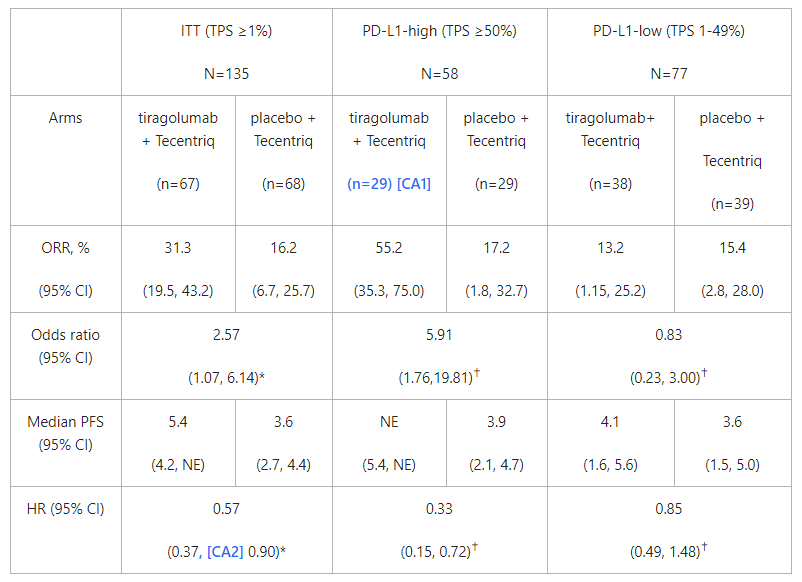
At the primary analysis, tiragolumab plus Tecentriq met both co-primary endpoints in the intention-to-treat (ITT) population, showing an improvement in the objective response rate (ORR) (31.3% versus 16.2%) and a 43% reduction in the risk of disease worsening or death (progression-free survival; PFS) (median PFS=5.4 versus 3.6 months; hazard ratio [HR]=0.57, 95% CI: 0.37–0.90) compared with Tecentriq alone.
An exploratory analysis in people with high levels of PD-L1 (TPS ≥50%) showed a clinically meaningful improvement in ORR (55.2% versus 17.2%) and a 67% reduction in the risk of disease worsening or death (median PFS=not reached versus 3.9 months; HR=0.33, 95% CI: 0.15–0.72) with the combination compared with Tecentriq alone.
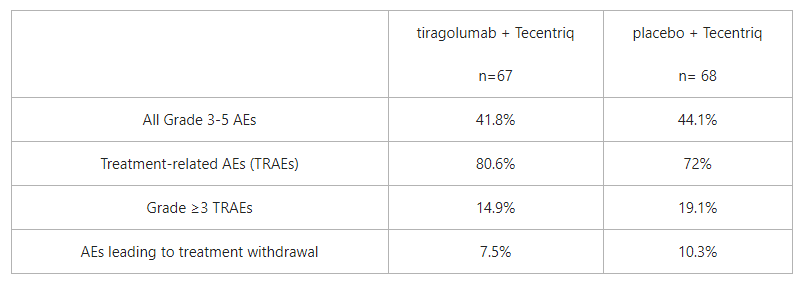
The data suggest that the combination of tiragolumab plus Tecentriq was well-tolerated, showing similar rates of all Grade 3 or more all-cause adverse events (AEs) when combining the two immunotherapies compared with Tecentriq alone (41.8% versus 44.1%).
At a six-month follow-up, the improvement in the ORR and PFS in the tiragolumab plus Tecentriq arm persisted in both the ITT and the PD-L1-high populations, and no new safety signals were observed.
As part of Genentech’s commitment to explore new immunotherapy options and combinations, the company recently initiated two Phase III clinical trials evaluating tiragolumab plus Tecentriq for people with certain types of lung cancer (SKYSCRAPER-01 and SKYSCRAPER-02). Tiragolumab is also being evaluated in other solid tumors as well as in hematological cancers. Additional Phase Ia/b results in solid tumors will be presented at an upcoming medical meeting.
About CITYSCAPE study
CITYSCAPE is a global Phase II, randomized and blinded study evaluating tiragolumab plus Tecentriq compared with Tecentriq alone in 135 patients with first-line PD-L1-positive, locally advanced unresectable or metastatic non-small cell lung cancer. Patients were randomized 1:1 to receive either tiragolumab plus Tecentriq or placebo plus Tecentriq, until progressive disease or loss of clinical benefit. Co-primary endpoints are objective response rate (ORR) and progression-free survival (PFS). Secondary endpoints include safety and overall survival (OS).
Efficacy results
*stratified
unstratified
At a six-month follow-up, the improvement in ORR (37.3% versus 20.6%) and PFS (median PFS=5.6 months versus 3.9 months) in the tiragolumab plus Tecentriq arm persisted in the ITT population. Results in the PD-L1-high population were also consistent with the first analysis and the median PFS was still not reached.
Safety results
About tiragolumab
Tiragolumab is a monoclonal antibody designed to bind with TIGIT, a protein receptor on immune cells. By binding to TIGIT, tiragolumab blocks its interaction with a protein called poliovirus receptor (PVR, or CD155) that can suppress the body’s immune response. Blockade of TIGIT and PD-L1 may synergistically enable the re-activation of T cells and enhance NK cell antitumor activity.
About Tecentriq® (atezolizumab)
Tecentriq is a monoclonal antibody designed to bind with a protein called PD-L1. Tecentriq is designed to bind to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, blocking its interactions with both PD-1 and B7.1 receptors. By inhibiting PD-L1, Tecentriq may enable the re-activation of T cells. Tecentriq may also affect normal cells.
About Genentech in cancer immunotherapy
Genentech has been developing medicines to redefine treatment in oncology for more than 35 years, and today, realizing the full potential of cancer immunotherapy is a major area of focus. With more than 20 immunotherapy molecules in development, Genentech is investigating the potential benefits of immunotherapy alone, and in combination with various chemotherapies, targeted therapies and other immunotherapies with the goal of providing each person with a treatment tailored to harness their own unique immune system.
In addition to Genentech’s approved PD-L1 checkpoint inhibitor, the company’s broad cancer immunotherapy pipeline includes other checkpoint inhibitors, individualized neoantigen therapies and T cell bispecific antibodies. For more information visit http://www.gene.com/cancer-immunotherapy.
About Genentech in lung cancer
Lung cancer is a major area of focus and investment for Genentech, and we are committed to developing new approaches, medicines and tests that can help people with this deadly disease. Our goal is to provide an effective treatment option for every person diagnosed with lung cancer. We currently have five approved medicines to treat certain kinds of lung cancer and more than 10 medicines being developed to target the most common genetic drivers of lung cancer or to boost the immune system to combat the disease.
About Genentech
Founded more than 40 years ago, Genentech is a leading biotechnology company that discovers, develops, manufactures and commercializes medicines to treat patients with serious and life-threatening medical conditions. The company, a member of the Roche Group, has headquarters in South San Francisco, California. For additional information about the company, please visit http://www.gene.com.
Source: Genentech
Posted: May 2020

