Alnylam Reports Positive Topline Results from ILLUMINATE-A Phase 3 Study of Lumasiran for the Treatment of Primary Hyperoxaluria Type 1
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Dec. 17, 2019 -- Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), the leading RNAi therapeutics company, announced today that the ILLUMINATE-A Phase 3 study of lumasiran, an investigational RNAi therapeutic targeting glycolate oxidase (GO) in development for the treatment of primary hyperoxaluria type 1 (PH1), met its primary efficacy endpoint and all tested secondary endpoints. Specifically, lumasiran met the primary efficacy endpoint of percent change from baseline, relative to placebo, in 24-hour urinary oxalate excretion averaged across months 3 to 6 (p less than 0.0001). The study also achieved statistically significant results for all six tested secondary endpoints (p less than or equal to 0.001). Lumasiran also demonstrated an encouraging safety and tolerability profile. Based on these results, the Company plans to submit a New Drug Application (NDA) and file a Marketing Authorisation Application (MAA) for lumasiran in early 2020.
“We are very pleased to report positive topline Phase 3 results for lumasiran, our third wholly owned investigational RNAi therapeutic. Patients living with PH1 and their families are faced with the burden of recurrent and painful stone events and a progressive and unpredictable decline in kidney function that ultimately results in end-stage renal disease and the need for intensive dialysis as a bridge to dual liver/kidney transplantation. The results from ILLUMINATE-A demonstrate that lumasiran can significantly reduce the hepatic production of oxalate, which we believe can thereby address the underlying pathophysiology of PH1,” said Akshay Vaishnaw, M.D., Ph.D., President of R&D at Alnylam. “Further, we are encouraged by the safety and tolerability profile of lumasiran and believe this investigational medicine has the potential to have a meaningful clinical impact on patients living with PH1. We look forward to submitting regulatory filings in early 2020 and advancing this highly needed medicine one step closer to patients. Finally, we extend our deepest gratitude to the patients, caregivers, investigators, and study staff who participated in ILLUMINATE-A and contributed to what we believe is an important medical advance for the treatment of PH1.”
“The ILLUMINATE-A results represent a significant landmark for the PH1 patient community. These patients live with the angst of not knowing when that next kidney stone will come or for how long their kidneys will keep working, and they grapple with the possibility of needing new organs. We have lived with the hope that someday patients living with PH1 and their families would finally have a treatment with the potential to have a positive impact on their health and alleviate some of that angst,” said Kim Hollander, Executive Director of the Oxalosis and Hyperoxaluria Foundation. “Today we are hopeful that we are much closer to that day than we have ever been.”
“Lumasiran results in ILLUMINATE-A mark our third positive Phase 3 study readout in 2019, positioning Alnylam with the potential for four marketed products by the end of 2020, assuming positive regulatory reviews. We believe this achievement also provides further support of our relatively high product development success rate linked to selection of genetically validated targets and a modular and reproducible platform,” said John Maraganore, Ph.D., Chief Executive Officer of Alnylam. “With these results in hand, we believe that we’re on track to exceed our Alnylam 2020 guidance, building – by the end of 2020 – a global, multi-product, commercial-stage company with a robust portfolio of clinical-stage programs for future growth and an organic product engine for sustainable innovation and patient impact.”
ILLUMINATE-A Topline Study Results
ILLUMINATE-A (NCT03681184), a randomized, double-blind, placebo-controlled trial, designed to enroll approximately 30 patients with PH1 ages six and above, at 16 study sites, in eight countries around the world, is the largest interventional study conducted specifically in PH1. Patients were randomized 2:1 to lumasiran or placebo, with lumasiran administered at 3 mg/kg monthly for three months followed by quarterly maintenance doses. The primary endpoint for the study was the percent change from baseline in 24-hour urinary oxalate excretion averaged across months 3 to 6 in patients treated with lumasiran as compared to placebo. At six months, lumasiran met the primary endpoint in patients with PH1 (p less than 0.0001) and achieved statistically significant results for all six hierarchically-tested secondary endpoints (p less than or equal to 0.001), including the proportion of lumasiran patients that achieved near-normalization or normalization of urinary oxalate levels, relative to placebo.
There were no serious or severe adverse events in the study, and results showed that lumasiran was generally well tolerated with an overall profile generally consistent with that observed in Phase 1/2 and open-label extension studies of lumasiran. Lumasiran has received U.S. and EU Orphan Drug Designations, Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA), and a Priority Medicines (PRIME) designation from the European Medicines Agency (EMA). Full ILLUMINATE-A study results will be presented in an oral plenary session on Tuesday, March 31, 2020 at OxalEurope International Congress in Amsterdam, Netherlands.
The Company is also conducting ILLUMINATE-B – a global Phase 3 study of lumasiran in PH1 patients less than six years of age, with results expected in mid-2020, and ILLUMINATE-C – a global Phase 3 study of lumasiran in PH1 patients of all ages with advanced renal disease, with results expected in 2021.
About Lumasiran
Lumasiran is an investigational, subcutaneously administered RNAi therapeutic targeting hydroxyacid oxidase 1 (HAO1) in development for the treatment of primary hyperoxaluria type 1 (PH1). HAO1 encodes glycolate oxidase (GO). Thus, by silencing HAO1 and depleting the GO enzyme, lumasiran inhibits production of oxalate – the metabolite that directly contributes to the pathophysiology of PH1. Lumasiran utilizes Alnylam's Enhanced Stabilization Chemistry (ESC)-GalNAc-conjugate technology, which enables subcutaneous dosing with increased potency and durability and a wide therapeutic index. Lumasiran has received both U.S. and EU Orphan Drug Designations, a Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA), and a Priority Medicines (PRIME) designation from the European Medicines Agency (EMA). The safety and efficacy of lumasiran have not been evaluated by the FDA, EMA or any other health authority.
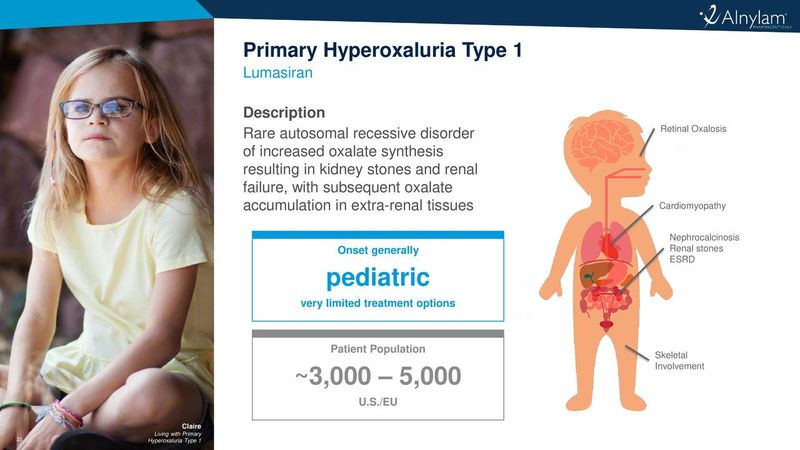
About Primary Hyperoxaluria Type 1 (PH1)
PH1 is an ultra-rare disease in which excessive oxalate production results in the deposition of calcium oxalate crystals in the kidneys and urinary tract and can lead to the formation of painful and recurrent kidney stones and nephrocalcinosis. Renal damage is caused by a combination of tubular toxicity from oxalate, calcium oxalate deposition in the kidneys, and urinary obstruction by calcium oxalate stones. Compromised kidney function exacerbates the disease as the excess oxalate can no longer be effectively excreted, resulting in subsequent accumulation and crystallization in bones, eyes, skin, and heart, leading to severe illness and death. Current treatment options are very limited and include frequent renal dialysis or combined organ transplantation of liver and kidney, a procedure with high morbidity that is limited due to organ availability. Although a small minority of patients respond to Vitamin B6 therapy, there are no approved pharmaceutical therapies for PH1.
About RNAi
RNAi (RNA interference) is a natural cellular process of gene silencing that represents one of the most promising and rapidly advancing frontiers in biology and drug development today. Its discovery has been heralded as “a major scientific breakthrough that happens once every decade or so,” and was recognized with the award of the 2006 Nobel Prize for Physiology or Medicine. By harnessing the natural biological process of RNAi occurring in our cells, a new class of medicines, known as RNAi therapeutics, is now a reality. Small interfering RNA (siRNA), the molecules that mediate RNAi and comprise Alnylam's RNAi therapeutic platform, function upstream of today’s medicines by potently silencing messenger RNA (mRNA) – the genetic precursors – that encode for disease-causing proteins, thus preventing them from being made. This is a revolutionary approach with the potential to transform the care of patients with genetic and other diseases. (Article from : www.drugs.com)

