Phathom Pharmaceuticals Announces Initiation of Pivotal Phase 3 Clinical Trial for Vonoprazan in Helicobacter Pylori (H. pylori) Infection
BUFFALO GROVE, Ill.--(BUSINESS WIRE)--Dec. 23, 2019 -- Phathom Pharmaceuticals, Inc. (Nasdaq: PHAT), a late clinical-stage biopharmaceutical company focused on developing and commercializing novel treatments for gastrointestinal diseases, announced today the initiation of PHALCON-HP. In this pivotal Phase 3 clinical trial, clinicians will evaluate vonoprazan in combination with amoxicillin (vonoprazan dual therapy) and vonoprazan in combination with amoxicillin and clarithromycin (vonoprazan triple therapy) for the successful eradication of H. pylori infection. With the initiation of PHALCON-HP, vonoprazan is now being evaluated in two pivotal trials to support regulatory submissions in two different disease areas in the United States and Europe. Earlier this month, the company initiated PHALCON-EE, a pivotal trial evaluating vonoprazan for both the healing and maintenance of healing of erosive esophagitis as well as the relief of heartburn. Topline data from both PHALCON-HP and PHALCON-EE are expected in 2021.
PHALCON-HP is a randomized, multicenter, Phase 3 trial that is planned to enroll approximately 975 patients with H. pylori infection. Participants will be randomized 1:1:1 to one of three arms: vonoprazan 20 mg administered twice a day (BID) and amoxicillin 1g administered three times a day (TID); vonoprazan 20 mg BID, amoxicillin 1 g BID and clarithromycin 500 mg BID; and lansoprazole 30 mg BID, amoxicillin 1 g BID and clarithromycin 500 mg BID. Each treatment regimen will be administered for 14 days. The primary endpoint of PHALCON-HP is the percentage of patients with successful eradication of H. pylori infection.
“The initiation of the PHALCON-HP study is another significant step in the development of vonoprazan for underserved patients living with gastrointestinal diseases,” said Azmi Nabulsi, MD, Chief Operating Officer of Phathom. “There are millions of people living with H. pylori infection in the United States and Europe and due to increased antibiotic resistance, eradication rates for patients treated with currently available therapies are declining. In addition to the troubling symptoms of H. pylori infection, H. pylori patients have an increased risk of developing other serious diseases including gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma. Vonoprazan, if approved, represents a first-in-class treatment that we believe will be a much needed new therapeutic option for H. pylori patients.”
About Helicobacter pylori (H. pylori) infection
H. pylori is a bacterial pathogen that is estimated to infect over 200 million individuals in the United States and Europe. As a result of the chronic inflammation induced by H. pylori infection, approximately 20% of infected patients develop a range of pathologies including dyspepsia, peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma. Gastric cancer is the third most common cause of cancer-related death worldwide, and over 80% of gastric cancers are attributed to H. pylori infection.
About Vonoprazan
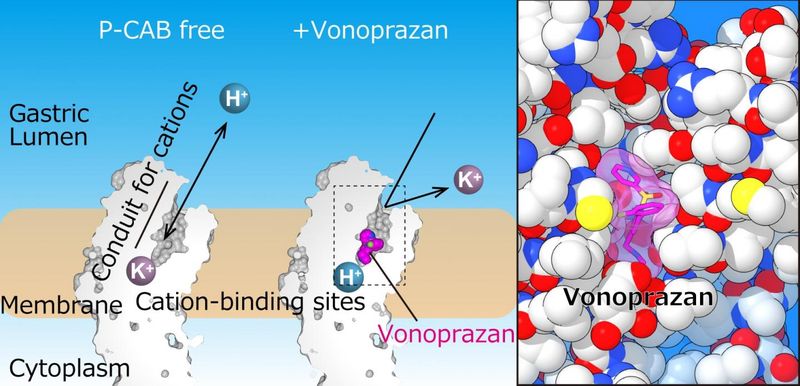
Vonoprazan, is an oral small molecule potassium competitive acid blocker (P-CAB). P-CABs are a novel class of medicines that block acid secretion in the stomach. Vonoprazan has shown rapid, potent, and durable anti-secretory effects and has demonstrated clinical benefits over standard of care treatments as a single agent in the treatment of gastroesophageal reflux disease (GERD), and in combination with antibiotics for the treatment of Helicobacter pylori (H. pylori) infection. The FDA has designated vonoprazan as a qualified infectious disease product (QIDP) and awarded Fast Track status for the treatment of H. pylori infection in combination with both amoxicillin and clarithromycin and with amoxicillin alone. Phathom in-licensed the U.S., European, and Canadian rights to vonoprazan from Takeda, which completed 17 Phase 3 trials for vonoprazan and received marketing approval in Japan and numerous other countries in Asia and Latin America. (Article from : www.drugs.com)

