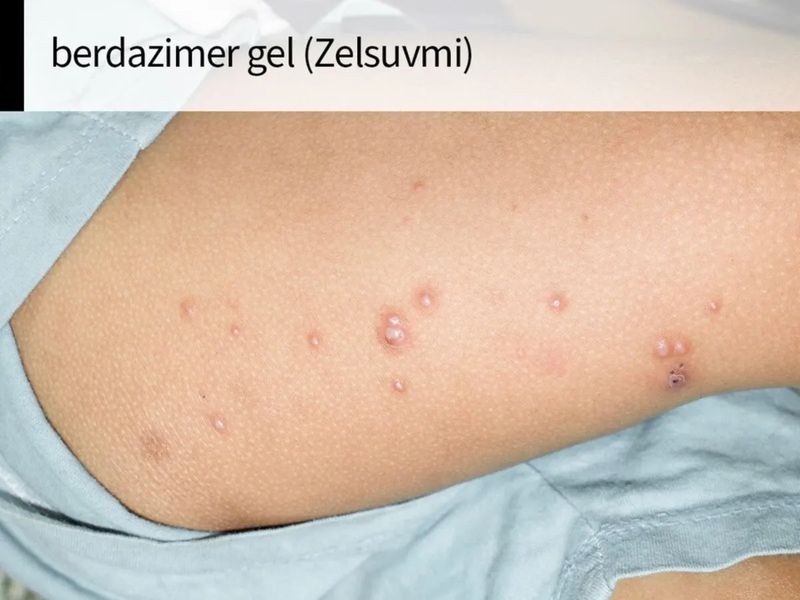
FDA Approves Zelsuvmi (berdazimer topical gel) for the Treatment of Molluscum Contagiosum
SAN DIEGO--(BUSINESS WIRE) January 5, 2024 -- Ligand Pharmaceuticals Incorporated (NASDAQ: LGND) today announced that the U.S. Food and Drug Administration (FDA) has approved Zelsuvmi™ (berdazimer topical gel, 10.3%) for the treatment of molluscum contagiosum (molluscum) in adults and pediatric patients one year of age and older.i The FDA approved Zelsuvmi as the first novel drug for the treatment of molluscum infections.
Zelsuvmi is the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home, outside of a physician's office, or other medical setting to treat this highly contagious viral skin infection.
“The approval of Zelsuvmi is a breakthrough, marking the first time that clinicians can treat molluscum with an efficacious topical prescription medication that is applied by the patient, or a family member,” said Mark D. Kaufmann, MD, FAAD, a Clinical Professor of Dermatology in the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City and Past President of the American Academy of Dermatology. “I look forward to having this novel medication to treat my molluscum patients.”
Molluscum is a highly contagious viral skin infection characterized by skin-colored to red lesions with a central, umbilicated viral core.ii Approximately 6 million Americans, primarily children, are infected each year.iii,iv However, up to 73% of children go untreated.v Treating the lesions is critical to preventing the viral infection from spreading to other people or to other areas of the body. vi
“It is nice to see that molluscum contagiosum is finally getting the attention it deserves. For those of us in the primary care field, it is wonderful to have an effective option that can be used at home rather than taking a wait and watch approach,” said Stephen W. Stripling, MD, Pediatrician, Study Investigator and Molluscum Researcher.
Zelsuvmi is a nitric oxide releasing agent. Nitric oxide has been shown to have antiviral properties.vii The mechanism of action of Zelsuvmi for the treatment of molluscum contagiosum is unknown. Nevertheless, Zelsuvmi's efficacy was demonstrated in 2 Phase 3 trials - B-SIMPLE 4 and B-SIMPLE 2. These trials showed Zelsuvmi's ability to reduce lesion counts and was well tolerated when used once a day.viii The B-SIMPLE Phase 3 program enrolled 1,598 patients.ix The most commonly reported adverse reactions (≥1%) in clinical trials were application site reactions. See additional Important Safety Information for Zelsuvmi below.
“We are proud of the team’s accomplishment, having completed the world’s largest clinical program in molluscum to bring this first-in-class topical medication to FDA approval,” said Todd Davis, CEO of Ligand. “Pediatricians, dermatologists, and caregivers have long-sought a convenient approach to treat this highly contagious skin infection. With Zelsuvmi, patients now have an at-home treatment option available.”
Zelsuvmi is expected to be available in the United States in the second half of 2024. Complete prescribing information is available at www.Zelsuvmi.com.
About Zelsuvmi™ (berdazimer topical gel, 10.3%)
Zelsuvmi (berdazimer topical gel, 10.3%) is a nitric oxide (NO) releasing agent indicated for the topical treatment of molluscum contagiosum in adults and pediatric patients one year of age and older. Complete prescribing information is available at www.Zelsuvmi.com.
Contraindications: None.
Warnings: Application site reactions, including, allergic contact dermatitis occurred. Discontinue Zelsuvmi and initiate appropriate therapy.
Adverse Reactions: The most commonly reported adverse reactions (≥1%) are application site reactions including pain such as burning or stinging sensations (18.7%), erythema (11.7%), pruritus (5.7%), exfoliation (5.0%), dermatitis (4.9%), swelling (3.5%), erosion (1.6%), discoloration (1.5%), vesicles (1.5%), irritation (1.2%), and infection (1.1%).
About Ligand Pharmaceuticals
Ligand is a biopharmaceutical company enabling scientific advancement through supporting the clinical development of high-value medicines. Ligand does this by providing financing, licensing our technologies or both. Our business model seeks to generate value for stockholders by creating a diversified portfolio of biotech and pharmaceutical product revenue streams that are supported by an efficient and low corporate cost structure. Our goal is to offer investors an opportunity to participate in the promise of the biotech industry in a profitable and diversified manner. Our business model is based on funding programs in mid- to late-stage drug development in return for economic rights and licensing our technology to help partners discover and develop medicines. We partner with other pharmaceutical companies to attempt to leverage what they do best (late-stage development, regulatory management and commercialization) in order to generate our revenue. Our Captisol® platform technology is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs. We have established multiple alliances, licenses and other business relationships with the world’s leading pharmaceutical companies including Amgen, Merck, Pfizer, Jazz, Takeda, Gilead Sciences and Baxter International. For more information, please visit www.ligand.com. Follow Ligand on X; (f/k/a Twitter) @Ligand_LGND.
We use our investor relations website and X as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Investors should monitor our website and our X account, in addition to following our press releases, SEC filings, public conference calls and webcasts.
Forward-Looking Statements
This news release contains forward-looking statements by Ligand that involve risks and uncertainties and reflect Ligand's judgment as of the date of this release. Words such as “plans,” “believes,” “expects,” “anticipates,” and “will,” and similar expressions, are intended to identify forward-looking statements. These forward-looking statements include: the timing of commercial launch of Zelsuvmi; the potential that Zelsuvmi could avoid surgical removal or more intensive therapies in some patients; and the potential market size of patients who can be treated with Zelsuvmi. Actual events or results may differ from Ligand's expectations due to risks and uncertainties inherent in Ligand’s business, including, without limitation: the risk that Ligand may not commercially launch Zelsuvmi in the second half of 2024 or at all; Ligand may not be able to successfully commercialize Zelsuvmi which will depend on a number of factors including coverage and reimbursement levels from governmental authorities and health insurers as well as market acceptance by healthcare providers; the market size for Zelsuvmi may be smaller than estimated; Ligand’s dependence on third parties in connection with product manufacturing and distribution of Zelsuvmi; Ligand may not be able to protect its intellectual property and patents covering Zelsuvmi which may be challenged or invalidated; and other risks described in Ligand’s prior press releases and filings with the Securities and Exchange Commission available at www.sec.gov. Ligand disclaims any intent or obligation to update these forward-looking statements beyond the date of this release. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
i Zelsuvmi Package Insert. LNHC Inc. 2023.
ii Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. Oct 2013;13(10):877-88. doi:10.1016/s1473-3099(13)70109-9
iii Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. Apr 2014;31(2):130-6. doi:10.1093/fampra/cmt075
iv United States Census Bureau. United States population by age and sex. Accessed October 30, 2023. https://www.census.gov/popclock/data_tables.php?component=pyramid
v Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? Experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. May-Jun 2015;32(3):353-7. doi:10.1111/pde.12504
vi Han H, Smythe C, Yousefian F, Berman B. Molluscum Contagiosum Virus Evasion of Immune Surveillance: A Review. J Drugs Dermatol. Feb 1, 2023;22(2):182-189. doi:10.36849/jdd.7230
vii Ward BM, Riccio DA, Cartwright M, Maeda-Chubachi T. Anitviral effect of berdazimer sodium on molluscum contagiosum virus using a novel in vitro methodology. Viruses. 2023, 15(12), 2360; https://doi.org/10.3390/v15122360
viii Browning JC, Enloe C, Cartwright M, et al. Efficacy and safety of topical nitric oxide−releasing berdazimer gel in patients with molluscum contagiosum: a phase 3 randomized clinical trial. JAMA Dermatol. 2022;158(8):871-878
ix Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2023;doi:https://doi.org/10.1016/j.jaad.2023.09.066https://www.jaad.org/article/S0190-9622(23)02890-6/fulltext
Source: Ligand Pharmaceuticals Incorporated
Posted: January 2024