Nirsevimab MEDLEY Phase II/III Trial Demonstrated Favourable Safety and Tolerability Profile in Infants at High Risk of RSV
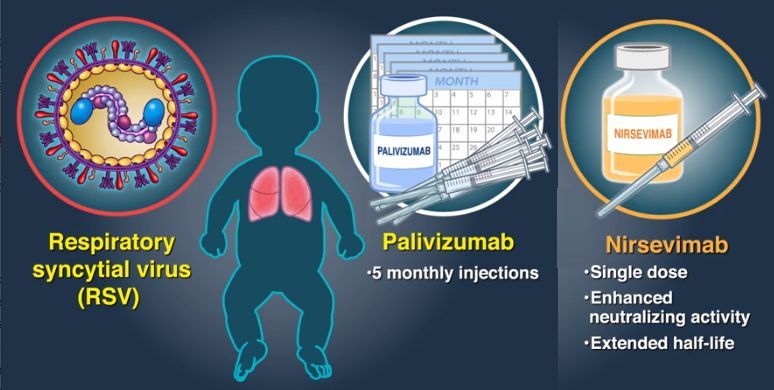
28 June 2021 -- The MEDLEY Phase II/III trial evaluated the safety and tolerability of nirsevimab compared to Synagis (palivizumab) when given to infants at high risk of respiratory syncytial virus (RSV) entering their first RSV season.1
The trial assessed the safety of nirsevimab in infants with chronic lung disease (CLD), congenital heart disease (CHD) and/or prematurity. Occurrence of treatment emergent adverse events (TEAEs) or treatment emergent serious adverse events (TESAEs) were similar between groups.1
Nirsevimab is a long-acting antibody, using AstraZeneca’s proprietary YTE technology, being developed by AstraZeneca and Sanofi with the potential to provide immunity directly to all infants and offer immediate protection against RSV through the entire season with a single dose.
Dr Joseph Domachowske, Professor of Pediatrics and Professor of Microbiology and Immunology at the State University of New York, Upstate Medical Center, Syracuse, NY and MEDLEY trial primary investigator, said: “These data for nirsevimab are important as they show a safety and tolerability profile comparable to the only available preventative option against lower respiratory tract infections caused by respiratory syncytial virus for preterm infants and those with health conditions. Given the typical respiratory syncytial virus season lasts nearly five months, there is a potential advantage to providing a preventative option that could help protect all infants with one dose for the entire season.”
Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, said: “Respiratory syncytial virus is the leading cause of hospitalisations in infants. These results, combined with the recent positive efficacy outcome of our MELODY Phase III trial and our Phase IIb data, contribute to the body of evidence demonstrating nirsevimab’s potential to protect all infants against respiratory syncytial virus with one dose. We look forward to sharing the results with regulators.”
Jean-François Toussaint, Global Head of Research and Development, Sanofi Pasteur: “Respiratory syncytial virus is the major remaining paediatric infectious disease with no preventative option available to all infants. We believe nirsevimab has the potential to become an important and innovative routine immunisation for all infants – those born prematurely or at term, healthy or with health conditions.”
Full results from the MEDLEY trial will be presented at a forthcoming medical meeting. The trial is ongoing to collect additional safety data.
Nirsevimab is also being evaluated in the MELODY Phase III trial, which met its primary endpoint of a statistically significant reduction in the incidence of medically attended LRTI caused by RSV, compared to placebo, in healthy late preterm and term infants during their first RSV season. MEDLEY, MELODY and the Phase IIb trial will form the basis of AstraZeneca’s regulatory submissions planned from the first half of 2022.
Nirsevimab has been granted breakthrough designation by three major regulatory agencies around the world. These include Breakthrough Therapy Designation by The China Center for Drug Evaluation under the National Medical Products Administration; Breakthrough Therapy Designation from the US Food and Drug Administration; and access granted to the European Medicines Agency PRIority MEdicines (PRIME) scheme.
RSV
RSV is a common, contagious pathogen that causes seasonal epidemics of lower respiratory tract infections (LRTI), including bronchiolitis and pneumonia.2-4 It is the leading cause of hospitalisations in infants worldwide.4 Currently, Synagis is the only approved option for the prevention of serious LRTI caused by RSV in preterm infants and infants at high risk and requires up to five injections to cover a typical RSV season.5 Globally, in 2015, there were approximately 30 million cases of acute lower respiratory infections leading to more than three million hospitalisations, and it was estimated that there were 60,000 in-hospital deaths of children younger than five years.4,6 Most hospitalisations for RSV occur in otherwise healthy infants born at term.7-11 Medically attended LRTIs are associated with increased costs to the healthcare system.12
MEDLEY
MEDLEY is a Phase II/III, randomised, double-blind, Synagis-controlled trial with the primary objective of assessing safety and tolerability for nirsevimab in preterm infants and infants at high risk eligible to receive Synagis. Between July 2019 and May 2021 approximately 925 infants entering their first RSV season were dosed with either nirsevimab or Synagis. Safety is assessed by monitoring the occurrence of TEAEs and TESAEs through 360 days post-dose. High-risk infants are defined as infants 35 weeks 0 days or lesser gestational age without CLD/CHD, or infants with CLD of prematurity or hemodynamically significant CHD.1
The evaluation of the safety and tolerability of nirsevimab in the MEDLEY trial was carried out earlier than anticipated. A primary analysis was conducted to allow earlier assessment of nirsevimab’s safety and tolerability versus Synagis based on a sufficient number of infants being enrolled and followed through their first RSV season.
MELODY
MELODY is a randomised, placebo-controlled Phase III trial conducted across at least 21 countries designed to determine the incidence of medically attended LRTI due to RSV confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) testing through 150 days after dosing, versus placebo, in healthy infants entering their first RSV season. Healthy late preterm and term infants born at 35 weeks 0 days or greater gestational age were randomised (2:1) to receive a single 50mg (in infants weighing <5kg) or 100mg (in infants weighing >5kg) intramuscular injection of nirsevimab or placebo. Between July 2019 and February 2021 approximately 1,500 infants were dosed with either nirsevimab or placebo at the RSV season start.13 An additional 1,500 infants will be enrolled in the Northern and Southern Hemispheres to complete the safety evaluation.
Nirsevimab
Nirsevimab is a long-acting antibody being developed as a passive immunisation for the prevention of LRTI caused by RSV. It is being developed for use with all infants, in comparison to the current standard of care, Synagis, which is limited to infants at high risk.5, 14,15 Due to its extended half-life technology, nirsevimab may only require one dose during a typical five-month RSV season.15 The current anti-RSV antibody, AstraZeneca’s Synagis, is limited to high-risk infants and provides one-month protection, requiring five injections to cover an RSV season.5
Nirsevimab is a passive immunisation, designed to provide RSV protection to all infants, whereby an antibody is given directly to an infant to help prevent RSV, unlike active immunisation, where the infants’ own immune system is activated to prevent or fight infection.16 Passive immunisation could offer immediate protection unlike active immunisation, which can take weeks to develop protection.16
In March 2017, AstraZeneca and Sanofi announced an agreement to develop and commercialise nirsevimab. Under the terms of the agreement, AstraZeneca leads all development and manufacturing activities and Sanofi will lead commercialisation activities and record revenues. Under the terms of the global agreement, Sanofi made an upfront payment of €120m, has paid a development milestone of €30m and will pay up to a further €465m upon achievement of certain development and sales-related milestones. The two companies share all costs and profits. Revenue from the agreement is reported as Collaboration Revenue in the Company’s financial statements.
Related, in November 2018, AstraZeneca divested US commercial rights for Synagis to Swedish Orphan Biovitrum AB (publ) (Sobi) in addition to the right to participate in payments that may be received by AstraZeneca from the US profits or losses for nirsevimab. Under the agreement, AstraZeneca received upfront consideration of $1.5bn, consisting of $1.0bn in cash and $500m in ordinary shares of Sobi upon completion, and will have received a total of $60m in non-contingent payments for nirsevimab during 2019-2021. AstraZeneca will also receive up to $470m in sales-related payments for Synagis, a $175m milestone following the submission of the Biologics License Application (BLA) for nirsevimab and potential net payments of approximately $110m on achievement of other nirsevimab profit and development-related milestones. Upon payment of the $175m milestone on BLA submission, Sobi’s ongoing participation will amount to AstraZeneca’s full share of profits or losses under the agreement with Sanofi for nirsevimab in the US. AstraZeneca will continue to manufacture and supply nirsevimab globally and is entitled to an additional royalty from Sobi if profits from nirsevimab in the US exceed a pre-specified level.
AstraZeneca in Respiratory & Immunology
Respiratory & Immunology, part of BioPharmaceuticals, is one of AstraZeneca’s three disease areas and is a key growth driver for the Company.
AstraZeneca is an established leader in respiratory care with a 50-year heritage. The Company aims to transform the treatment of asthma and COPD by focusing on earlier biology-led treatment, eliminating preventable asthma attacks, and removing COPD as a top-three leading cause of death. The Company’s early respiratory research is focused on emerging science involving immune mechanisms, lung damage and abnormal cell-repair processes in disease and neuronal dysfunction.
With common pathways and underlying disease drivers across respiratory and immunology, AstraZeneca is following the science from chronic lung diseases to immunology-driven disease areas. The Company’s growing presence in immunology is focused on five mid- to late-stage franchises with multi-disease potential, in areas including rheumatology (including systemic lupus erythematosus), dermatology, gastroenterology, and systemic eosinophilic-driven diseases. AstraZeneca’s ambition in Respiratory & Immunology is to achieve disease modification and durable remission for millions of patients worldwide.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
References
1. Clinicaltrials.gov. A Study to Evaluate the Safety of MEDI8897 for the Prevention of Medically Attended Respiratory Syncytial Virus (RSV) Lower Respiratory Track Infection (LRTI) in High-risk Children. https://clinicaltrials.gov/ct2/show/NCT03959488. Accessed June 2021.
2. Piedimonte G, Perez MK. Pediatr Rev. 2014;35(12):519-53
3. Oymar K et al. Scand J Trauma Resusc Emerg Med. 2014;22:23
4. Shi T, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–58.
5. Synagis (palivizumab) injection prescribing information. Available at: https://www.synagis.com/synagis.pdf. Accessed June 2021.
6. Oxford Vaccines Group. What is RSV? https://vk.ovg.ox.ac.uk/vk/rsv. Accessed April 2021
7. Hall CB, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341-e348.
8. Rha B, et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015-2016. Pediatrics. 2020;146:e20193611.
9. Arriola CS, et al. Estimated Burden of Community-Onset Respiratory Syncytial Virus-Associated Hospitalizations Among Children Aged <2 Years in the United States, 2014-15. J Pediatric Infect Dis Soc. 2020;9:587-595
10. Krilov LR, et al. Impact of the 2014 American Academy of Pediatrics Immunoprophylaxis Policy on the Rate, Severity, and Cost of Respiratory Syncytial Virus Hospitalizations among Preterm Infants. Am J Perinatol. 2020;37:174-183.
11. Scheltema NM, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5:e984-9.
12. Zhang S et al. J Infect Dis. 2020. doi: 10.1093/infdis/jiz683
13. Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended RSV LRTI in Healthy Late Preterm and Term Infants (MELODY). https://clinicaltrials.gov/ct2/show/NCT03979313. Accessed June 2021.
14. Villafana T, et al. Passive and active immunization against respiratory syncytial virus for the young and old. Expert Rev Vaccines. 2017;16:1-39.
15. Zhu Q, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9:pii: eaaj1928
16. Centers for Disease Control and Prevention. Vaccines & Immunizations. August 18, 2017. https://www.cdc.gov/vaccines/vac-gen/immunity-types.htm. Accessed June 2021.
Source: AstraZeneca
Posted: June 2021

