Bristol Myers Squibb and The Rockefeller University Announce License Agreement for SARS-CoV-2 Neutralizing Monoclonal Antibody Combination for the Treatment of COVID-19
NEW YORK--(BUSINESS WIRE) February 3, 2021 -- Bristol Myers Squibb (NYSE: BMY) and The Rockefeller University today announced that they have entered into a definitive agreement under which Bristol Myers Squibb has been granted a global exclusive license to develop, manufacture and commercialize Rockefeller’s novel monoclonal antibody (“mAb”) duo treatment that neutralizes the SARS-CoV-2 virus for therapy or prevention of COVID-19.
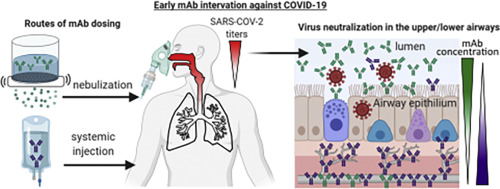
Despite the increasing availability of the vaccines, there will continue to be patients who contract COVID-19 and will need treatment for their infection. This novel treatment is a combination of two mAbs directed at blocking the SARS-CoV-2 spike protein and neutralizing the virus. The mAbs have been engineered to be highly potent and stable, allowing them to last longer in the bloodstream. Preclinical data suggest that this could enable effective treatment against multiple variants of the virus using a low dose subcutaneous administration, which would increase access to the medicine by eliminating the need for intravenous infusion.
Ultimately, should the clinical development be successful, these advantages could potentially help expand access globally, including to low- and middle-income countries and to communities where healthcare resources are limited—a goal that both institutions will jointly work towards.
Phase 1 clinical trials to assess dosing for IV and subcutaneous formulations, and to assess safety for the mAb duo, were initiated by Rockefeller in mid-January. Planning is underway with the goal of moving rapidly to a registrational program following readout of the phase 1 study taking place at Rockefeller University Hospital.
“We look forward to continuing to work with The Rockefeller University in this crucial effort,” said Ho Sung Cho, Ph.D., Senior Vice President, Discovery Biotherapeutics, Bristol Myers Squibb. “Bristol Myers Squibb has supported the development and manufacturing of clinical supplies for Rockefeller to begin the Phase 1 trial and we are committed to leveraging our capabilities and resources in an effort to expeditiously develop and bring this potential treatment to patients and further address the challenges of the pandemic.”
“The two highly potent antibodies against SARS-CoV-2, discovered by Rockefeller scientists, have the potential to play an important role in treating COVID-19 patients,” said Richard P. Lifton, President, The Rockefeller University. “New treatment options are urgently needed that treat mild to moderate disease and prevent development of severe disease in high-risk patients. Our collaboration with BMS will help us accelerate development timelines and support rapid delivery to patients.”
Included in the terms of the agreement, Rockefeller is entitled to receive royalty payments on future sales. Should the clinical development be successful, Bristol Myers Squibb will work to enable availability and affordability of this potential treatment to patients globally.
About SARS-CoV-2 mAb Combo
Rockefeller has discovered two complementary antibodies to the SARS-CoV-2 spike protein that synergistically neutralize the virus in vitro and in animal models. The two antibodies have shown activity against several known SARS-CoV-2 mutants and it is believed that their co-administration could reduce the possibility of mutant virus escape. The two antibodies are potentially unique as pertains to their long half-life in humans and high affinity for the spike protein. The longer half-life has been enabled by leveraging Xencor’s Fc engineering technology.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol Myers Squibb, visit us at BMS.com or follow us on LinkedIn, Twitter, YouTube, Facebook and Instagram.
Celgene and Juno Therapeutics are wholly owned subsidiaries of Bristol-Myers Squibb Company. In certain countries outside the U.S., due to local laws, Celgene and Juno Therapeutics are referred to as, Celgene, a Bristol Myers Squibb company and Juno Therapeutics, a Bristol Myers Squibb company.
About The Rockefeller University
The Rockefeller University is the world’s leading biomedical research university and is dedicated to conducting innovative, high-impact research to improve the understanding of life for the benefit of humanity. The university’s unique approach to science, with only 70 faculty, each selected for pursuit of highly innovative ideas, has led to many of the world’s most revolutionary and transformative contributions to biology and medicine. During Rockefeller’s 120-year history, Rockefeller scientists have won 26 Nobel Prizes, 24 Albert Lasker Medical Research Awards, and 20 National Medals of Science.
Source: Bristol Myers Squibb
Posted: February 2021

